UPDATE: On 11/09/2023, FDA released a notice that enforcement for product listing and facility registration requirements under MoCRA will be delayed by 6 months, making the new compliance deadline 07/01/2024. This update comes following delays to the release of the Cosmetic Direct platform.
As new cosmetic product regulation compliance deadlines are quickly approaching, SRC Senior Consultant, Crystal Maira, has provided answers to frequently asked questions our team has experienced over the past few months.
What Can I Do Now to Prepare for the December 29, 2023 Deadlines?
While the online platforms for Cosmetic Facility Registration and Product Listings are not expected to be available until late October, there are steps that each responsible person can take now to prepare for the upcoming MoCRA compliance deadlines.
Review and Comment on FDA’s draft electronic submission portal and paper forms
FDA has released a Draft Cosmetics Direct Electronic Submissions Portal slide deck and Draft FDA Forms 5066 – Registration of Product Facility and 5067 – Cosmetic Product Listing for public comment which provides a clear outline and step-by-step process for users to submit listings to FDA. Though paper forms are available, FDA is requesting that the online Cosmetics Direct portal be used whenever possible to maintain efficiency in the Agency’s review process.
FDA is asking for comments on the draft portal and forms, which can be submitted to FDA online at federalregister.gov until October 18, 2023.
SRC‘s review of the draft Cosmetics Direct portal found it to be substantially similar to the current process for submitting drug product listings and facility registrations through CDER Direct. We recommend reviewing the screenshots in the draft slide deck for how to submit product listings and facility registrations through the portal to help you estimate the resources you will need to complete all your product and facility listings prior to the December 29th deadline. SRC is available to do these required listings for companies and to act as the Authorized Agent for foreign entities.
Safety Substantiation
By 12/29/2023, persons responsible for cosmetic products must maintain records to support the safety substantiation of their products. These records can include studies, research, analysis, or any other records that scientific experts would reasonably consider sufficient to demonstrate the safety of the product.
SRC recommends companies begin researching and compiling the data necessary to defend the safety of their products, so they are prepared to comply before the deadline. Our scientific consultants are available to assist with data collection and review.
Adverse Event Reporting
Beginning on 12/29/2023, responsible persons must keep records of adverse events associated with all cosmetic products. Any serious adverse event (SAE) must be reported to FDA within 15 days of receiving the event record. FDA has confirmed that they will use the current MedWatch adverse event program for cosmetic events reporting.
SRC suggests that responsible persons start developing the process by which they will collect, review, and maintain adverse event reports, so they are prepared for the upcoming deadline. Our consultants will be available to help companies develop such policies and procedures and to submit SAE reports to FDA.
Professional Product Labeling
By 12/29/2023, professional cosmetic product labels must include a “clear and prominent statement that the product is administered or used only by licensed professionals”. SRC recommends updating product labels as soon as possible to assure the correct label is in the market prior to this deadline.
Which Facility Registration and Product Listing Requirements Apply to My Product?
As reviewed in a previous blog post Let’s Get Ready for MoCRA!, depending on the claims made about the product, a cosmetic product can be classified as either a cosmetic, a drug, or both under the Federal Food, Drug, & Cosmetics Act (FD&C). If an individual product is classified as both a drug and a cosmetic, the responsible person will only have to list the product and register that manufacturing facility under the FDA CDER (drug) platform.
The table below outlines the different product types that may be affected by MoCRA and what registration and listing requirements apply.
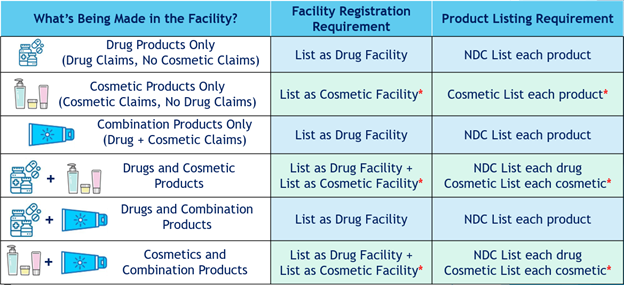
*Listing platform not yet available from FDA
Is “Soap” a Cosmetic?
The Federal Food, Drug, & Cosmetics Act (FD&C) defines a cosmetic as “a product, except soap, intended to be applied to the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance.” Many manufacturers call their products “soap”, which may lead them to believe that the regulations in MoCRA do not apply to their product. However, there is a very narrow legal definition of “soap” in the FD&C which states soap is “a product in which the non-volatile portion consists principally of an alkali salt of fatty acids”. The addition of any synthetic detergents in the soap would classify the product as a cosmetic.
Not only is the composition of the “soap” very limited, but the only claims allowed under the soap exclusion are for cleansing. Any claims of moisturizing or deodorizing would make the product a cosmetic which must meet all requirements outlined in MoCRA.
Based on this narrow definition and allowance, the vast majority of soap products are actually cosmetics and will require the persons responsible to meet the MoCRA requirements.
SRC offers full label and ingredients review services for cosmetic products. If you are unsure if your product qualifies under the exemption for “soap” in the FD&C, please contact your SRC consultant for assistance.
Contact SRC for a complimentary call to discuss how we may assist you with your cosmetic product compliance needs!

Crystal Maira
Senior Consultant
Published September 29, 2023


