On December 21, 2022, EPA published an Interim Guidance and two new methods to add bacterial and viral disinfection claims on porous surfaces. The guidance and methods may be used immediately for claim support, though registrants should be aware that EPA released the guidance with a 30-day comment period that was then extended through February 19th. This comment period may result in future changes.
The SRC Efficacy Team is preparing comments for submission to EPA and has created a summary of key highlights from our initial review.
- EPA has provided an interim guidance, a bacterial method and a viral method as well as initial data conducted by the EPA BEAD MLB laboratory. BEAD data confirms the feasibility and repeatability of the untreated control for S. aureus, P. aeruginosa, and Coronavirus 229E. Collaborative or Ring Trial efficacy data was not provided; therefore, the reproducibility of the methods is unknown. Efficacy data for the virus testing was not shared so the repeatability and reproducibility of the viral method is also unknown.
- The methods are based on the QM (previously known as the OECD Method). It uses 1cm diameter carriers made of “soft” or “flexible” porous surfaces that are suitable to be cut to this size/shape, and are able to withstand testing and sterilization. The carriers are cut by hand using a standard hole punch. A 10uL drop of inoculum plus Three-Part Soil (BSA, yeast extract, and mucin) is dried on the carrier and then exposed to 50uL of disinfectant. The carriers are neutralized, survivors are quantitatively recovered, and compared to control carriers to determine a log reduction (LR).
- The final test carriers are:
- PCF = 54% polyester/46% fire retardant polyester privacy curtain,
- NVF = Polyurethane faced fabric with polycarbonate/polyether resin with polyester backing, and
- VF = vinyl seating fabric with polyester backing.
- BEAD demonstrated 4.4 – 5.69 ± (0.09 – 0.24) bacterial log10/carrier and 4.0 – 5.0 ± (0.14 – 0.37) viral log10/carrier recoveries across the carrier types. This is excellent repeatability and recovery levels for these complex surfaces. The 100% cotton duck canvas was eliminated as the carrier counts occasionally had low recoveries. Additional surfaces may be considered but must meet the above quality and recovery.
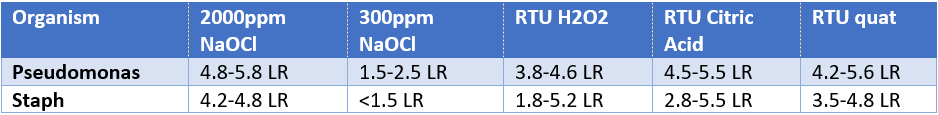
- Across the range of porous carriers, BEAD demonstrated the following results over 2-3 test dates using a 5 minute contact time with soil included:
-
- No viral data was provided.
- The method is not currently permitted for use to support claims for residential surfaces. It is for healthcare and institutional use surfaces only. SRC is hopeful that with provision of data showing the presence and frequency of these surfaces in residential settings that EPA may reconsider this stance.
- The guidance requires the product to have completed the required bacterial disinfection strains and each viral strain on hard, non-porous surfaces (810.2200) before the porous surface claims may be added. To have a viral porous disinfection claim, the bacterial porous disinfection claim must be completed. The guidance does not address products with porous claims only.
- All product forms are required to complete a wetness test per the C. difficile guidance.
- Additional bacteria and viruses must be tested to be claimed as no bridging is being allowed at this time.
- The claim is for public health pathogens only on non-clothing fabrics and textiles/upholstery where spot cleaning is the primary means of treatment. The claim is not intended for clothing, items that can be easily laundered, untreated wood, concrete or other hard porous materials, carpet/rugs, or the backing/stuffing material under the porous surface.
- The guidance/claim is not for residual claims, limited disinfectants, or tuberculocidal, fungal, yeast, or bacterial endospores claims. An EPA PRIA New Protocol review will be required for these microorganisms. At this time, EPA has not included Salmonella enterica testing to support a General/Broad Spectrum claim for non-healthcare use locations.
- The guidance/claim is for sprays (trigger, aerosols), liquids, and foams.
- Sprays and foams are sprayed into a container and then pipetted on the carrier. Foams are required to fully break before being pipetted onto the carriers.
- Towelettes, powders, fogs/mists, and ESS have been omitted from the Guidance and Agency consultation will be required. We are hopeful that EPA may reconsider these omissions following the comment period.
- These claims are eligible for List N or other lists and EVP claims where the appropriate strains are tested.
- EPA has defined Room Temperature in these methods at 21 ± 3°C (18°C – 24°C).
In summary, here are the requirements for bacterial and viral porous disinfection claims:
To support a porous bacterial disinfectant claim using the new EPA method:
- Prerequisite: Bacterial disinfectant (GST, UDM) for hard, non-porous surfaces (810.2200)
- Bacterial Test: S. aureus (ATCC 6538) and P. aeruginosa (ATCC 15442) on 3 LCL lots against the 3 carrier types/lot/strain
- Carrier counts must be 4.0 – 5.5 log10 CFU/carrier. No carriers may fall below 4.0 log10.
- 5 test carriers and 3 control carriers will be tested per carrier type/strain/lot.
- Three Part Soil (3PS) must be mixed with the test strains.
- Each lot must be tested on a separate day, but strains and carrier types may share a test date.
- For Additional Bacteria, replication may be reduced to 2 LCL lots. (Note: This is more stringent than hard, non-porous surface testing which allows NCL lots to be tested.)
- Performance Criteria: Mean 4.0 LR in≤10 mins ± 5 sec on all fabric types
- Compatibility test: Submit data and observations per the labeled use to demonstrate product compatibility with each porous carrier type to show physical degradation, pitting, fraying, cracking, delamination, bleaching, etc. Test methods were not specified.
- Wetness Test: https://www.epa.gov/pesticide-registration/methods-and-guidance-testing-efficacy-antimicrobial-products-against-spores
To support a porous viral disinfectant claim using the new EPA method:
- Prerequisites:
- Bacterial disinfectant (GST, UDM) for hard, non-porous and porous surfaces; and
- Viral disinfectant (E1053) for hard, non-porous claim on same virus intended for porous claim
- Viral Test: Hardest to kill virus of those intended to be claimed on 2 LCL lots against the 3 carrier types/lot/strain
- Carrier counts must be 4.0 – 5.5. log10/carrier. No carriers may fall below 4.0 log10.
- 5 test carriers and 3 control carriers will be tested per carrier type/strain/lot.
- Three Part Soil (3PS) must be mixed with the test strains. For viruses with high levels of soil in frozen stock, this will add an excessive amount of soil to the testing.
- Each lot must be tested on a separate day but strains and carrier types may share a test date.
- For Additional Virus, 2 NCL lots are permitted. Testing must be conducted on separate days.
- Performance Criteria: Mean 3.0 LR in ≤10mins ± 5 sec. on all fabric types
- Compatibility test: Submit data and observations per the labeled use to demonstrate product compatibility with each porous carrier type to show physical degradation, pitting, fraying, cracking, delamination, bleaching, etc. Test methods were not specified.
EPA has provided the following example directions for use and marketing claims:
Directions:
-
- “Apply in a limited area (spot treatment), monitor treated area for wetness for duration of the contact time, and allow to dry.
- Apply to surfaces only. Do not use on surfaces that routinely contact skin (i.e., clothing, sheets, towels).
- Only for use on non-launderable surfaces or those that may be laundered on an infrequent (non-routine) basis.”
Marketing claims:
-
- An effective disinfectant for the surface of porous materials*
- *Non-clothing fabrics, textiles, and upholstery that may be laundered on an infrequent (non-routine) basis where spot treatment is the primary means of cleaning and/or disinfection such as waiting room chairs, privacy curtains, safety belts, laboratory chairs, vehicle interiors (e.g., public transit, medical transportation), seat coverings, mattress covers not intended to be laundered, and mattress covers fixed to mattresses.”
- An effective disinfectant for the surface of porous materials*
Contact your SRC consultant for assistance with efficacy testing to add claims for use on porous materials.
 Rhonda Jones, RM(AAM)
Rhonda Jones, RM(AAM)
CEO
Posted: 01/06/2023


