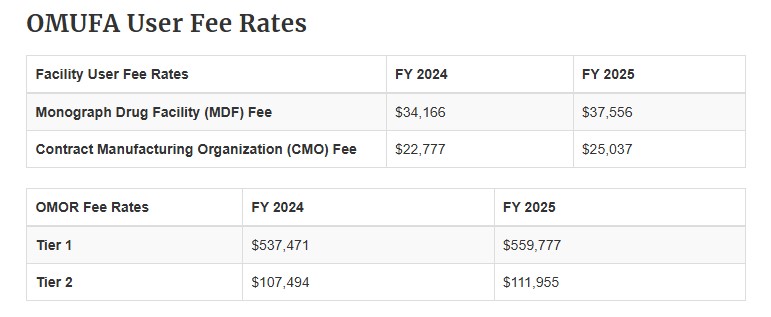
On March 20, 2025, the US Food and Drug Administration (FDA) announced its 2025 fees for OTC monograph drug facilities under the Over-the-Counter Monograph Drug User Fee Program (OMUFA). These fees are required to be paid by all “qualifying persons who own an OTC monograph drug facility, including contract manufacturing organization facilities”. This year, the Monograph Drug Facility (MDF) fee has increased by $3,390 and the Contract Manufacturing Organization (CMO) fee has increased by $2,260 (See below chart for 2024 and 2025 user fee rates). These changes equate to a nearly 10% increase over the previous year’s fees. The official Federal Register Notice was released on March 21, 2025 and details the considerations made when assessing this year’s fee, including total reported facility revenue, the number of MDF and CMO facilities, and an inflation adjustment of $1.2 million.
 *Screenshot from FDA Website
*Screenshot from FDA Website
OMUFA Facility fees are due on June 2, 2025. FDA will not send an invoice prior to the due date for these fees. To pay your OMUFA fees, follow the instructions on the FDA’s OTC OMUFA Monograph Facility Fee Cover Sheet Creation Process: Step-by-Step Instructions. Payment must be made in U.S. currency by electronic check or wire transfer, payable to the order of the Food and Drug Administration.
The SRC team is available to help you achieve and maintain compliance for your over-the-counter drug products. Please contact your SRC consultant if you have any questions or would like assistance with payment processing.

Bob MacDonald
VP of Regulatory Services
Posted 04/08/2025


