By Denise Fernadez, Ph.D.
Last updated on July 17, 2025
Upon the outbreak of Coronavirus Disease 2019 (COVID-19), consumers were instructed by the Centers for Disease Control and Prevention (CDC) to protect themselves and their families by washing their hands with soap and water for at least 20 seconds or utilizing a hand sanitizer containing at least 60% alcohol when handwashing is not available. CDC also recommended disinfecting “high touch” surfaces according to the disinfection use directions displayed on a product’s label. While disinfecting frequently touched surfaces seems relatively straightforward, very few disinfectants in the marketplace currently list SARS-CoV-2, the virus causing COVID-19, on the container label. If the virus is not listed on the label, then how does the consumer know which products may be effective in inactivating this new virus?
This question was quickly answered by the United States Environmental Protection Agency (U.S. EPA), when they triggered their Emerging Viral Pathogen Policy which allows information regarding products anticipated to be effective against SARS-CoV-2 to quickly be communicated via company websites, social media sites, technical literature to healthcare providers, and other off-label means of disseminating information.
What is the EPA Emerging Viral Pathogen Policy?
For a disinfectant product’s container label to list a specific virus, registrants must perform efficacy testing to demonstrate the product inactivates the virus, then submit and gain EPA acceptance of the data and label changes. When a new viral pathogen like SARS-CoV-2 emerges, the outbreak strain is not immediately available to efficacy testing laboratories to conduct studies. In addition, the newly emerged virus may not be compatible with the existing EPA-required test methodology for viral disinfection. These factors can result in a delay of testing disinfectant products against the newly emerged viral pathogen. However, once efficacy testing has been successfully completed and the study is accepted by the U.S. EPA, U.S. states and territories must then individually approve the new container label that lists the virus before the product can be marketed with the new viral claim. Taken together, these hurdles may potentially result in a delay of 17 months or longer between the time the product is tested against the new virus and the time users see the virus specifically listed on a product label. Disinfecting surfaces to inactivate the new viral pathogen is a necessity; users quickly seek information from the EPA regarding which products are recommended for use against the new viral pathogen and cannot wait months for approval processes to be completed.
Given the extensive time it takes from product testing to state approval, how are users (e.g., healthcare providers, general consumers, teachers, institutional workers, etc.) to proceed in the interim? The U.S. EPA’s solution is the Emerging Viral Pathogen Policy, which is built upon the Spaulding Classification model. This model creates a hierarchy of viruses based on their structure, which can be used to predict their susceptibility to disinfectant products. The policy allows registrants to make indirect claims on off-label communications against the emerging virus if they have demonstrated their product is effective against one or more viruses that are harder to inactivate than the emerging virus. Indirect claims convey to the user that the product is anticipated to be effective against the emerging viral pathogen based on a product’s demonstrated efficacy against viruses similar to the emerging virus.
How to Qualify for an Emerging Viral Pathogens Claim Against SARS-CoV-2
The Emerging Viral Pathogen guidance categorizes viruses into three categories, with enveloped viruses being the most susceptible to inactivation by a typical disinfectant and small, non-enveloped virus being the least susceptible, as follows:
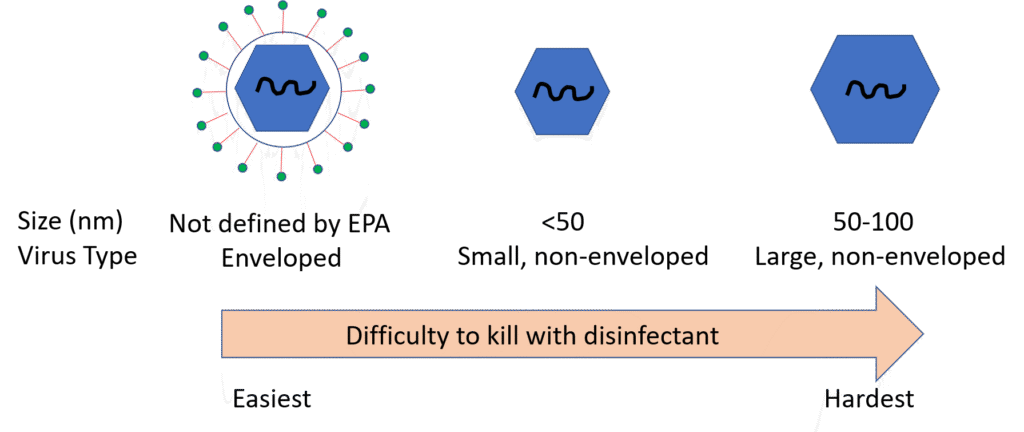
SARS-CoV-2, a member of the Coronaviridae family, is an enveloped virus. To qualify to make the allowed indirect claims against SARS-CoV-2 on off-label communications, a registration must be approved for an Emerging Viral Pathogen claim against at least one small or one large non-enveloped virus (please contact SRC for more information regarding virus classification). If an Emerging Viral Pathogen claim is approved by the EPA, the standard Emerging Viral Pathogen language will clearly be displayed on the disinfectant’s EPA Master Label. If a product satisfies the data requirements outlined in the Emerging Viral Pathogen policy but the EPA approved master label does not contain the Emerging Viral Pathogen language, a registrant may file an amendment with EPA to add these claims per Agency guidance. Either a PRIA or FQPA amendment can be used to add the Emerging Viral Pathogen language to the EPA master label. At this time, EPA is expediting review of EPA-registered disinfectants that are eligible to add the Emerging Viral Pathogen claim to their label and is also expediting review of studies which qualify a product for addition to List N or which have been directly tested against SARS-CoV-2.
Claim Communication Allowance
Once a disinfectant product’s Master Label is approved with an Emerging Viral Pathogen claim, registrants may proceed to make qualified indirect claims against the virus through off-label communications once the Emerging Viral Pathogen Policy has been triggered by the U.S. EPA. Acceptable off-label forms of communication include website and social media announcements, answering service tech calls, etc. The Emerging Viral Pathogen policy does not permit direct claims to be made against the emerging viral pathogen (e.g., SARS-CoV-2) on-label or off-label.
EPA has provided specific language to convey a product is anticipated to be effective against the emerging viral pathogen. The language used must follow one of the two formats provided in Attachment 1 of the “Emerging Viral Pathogen Program Guidance”. For example, XYZ Company may convey that “XYZ Disinfectant”, which has Emerging Viral Pathogen language approved on its EPA master label, is expected to be effective against SARS-CoV-2 via a LinkedIn post:
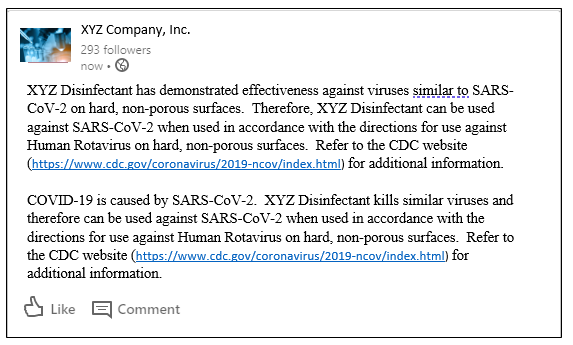
In this example, the Emerging Viral Pathogens claim against SARS-CoV-2 is supported by the large, non-enveloped Human Rotavirus data.
EPA List N: Disinfectants for Use Against SARS-CoV-2
To help users determine if disinfectant products meet the criteria for use against SARS-CoV-2, EPA has created List N – “Disinfectants for Use Against SARS-CoV-2”. In addition to products that have a qualifying emerging viral pathogen claim, EPA has now expanded the list to include products which have claims against (i) Human Coronavirus, (ii) Ebola virus (products found on EPA List L), (iii) Norovirus (products found on EPA List G), or other pathogens of similar difficulty. EPA has also recently allowed an emerging viral pathogen claim to be added based on C. difficile sporicidal data as spores are understood to be more difficult to disinfect than viruses.
If a qualified product is not already included on List N, registrants may provide their product name and EPA Registration Number to disinfectantlist@epa.gov. EPA continues to update the list on a weekly basis.
Other SARS-CoV-2 Disinfectant Lists
The Center for Biocide Chemistries (CBC) and Canada’s Natural and Non-Prescription Health Products Directorate (NNHPD) have each issued lists of disinfectants that are predicted to be effective against SARS-CoV-2.
To add an EPA-registered disinfectant to CBC’s List of Novel Coronavirus (COVID-19) – Fighting Products, the product name and EPA registration number should be provided to Komal Jain at komal_jain@americanchemistry.com. CBC will conduct a review of the product’s Master Label prior to adding a product to the list to ensure it meets EPA criteria for List N. In contrast to EPA’s List N, the CBC list includes all the brand names for a given EPA Registration which may be more easily recognized by users. The CBC list also includes brand names for all supplemental registrations.
Health Canada’s NNHPD should be contacted at hc.nnhpd-dpsnso.sc@canada.ca for more information on how to add a qualified disinfectant product to the Hard-surface disinfectants and hand sanitizers (COVID-19): List of disinfectants with evidence for use against COVID-19. NNHPD will continue to update the list as more products demonstrate that they are likely to be effective against SARS-CoV-2.
Direct Claims Against SARS-CoV-2
Currently, two U.S.-based contract laboratories with BSL-3 facilities offer testing of disinfectant products against SARS-CoV-2. EPA requires a product to achieve disinfection level performance against bacteria before a virucidal claim may be added to the label. EPA recently announced that they granted the first SARS-CoV-2 claims to several disinfectant products and are in the process of reviewing SARS-CoV-2 studies for numerous other products. Please contact SRC if you would like assistance with testing disinfectant, laundry, or other products against SARS-CoV-2.
References: